 |
|
|
In-licensed for the first time in Australia by Seqirus, a wholly-owned subsidiary of CSL, from leading ophthalmic company Santen Pharmaceutical Co Ltd, Ikervis contains the disease-modifying treatment ciclosporin, used to reduce inflammation associated with severe keratitis in DED.
According to Professor Stephanie Watson, Ophthalmologist, Clinician Scientist, Ophthalmic Surgeon and Chair of the Ophthalmic Research Institute of Australia, the COVID-19 pandemic is exacerbating DED cases due to increased mask use and screen time.
"We have seen a marked increase in dry eye symptoms among mask users. Typically, DED affects women more than men. According to a US study, women are almost three times more likely to develop DED than men and often progress to more severe forms of the disease earlier than men," said Prof. Watson.
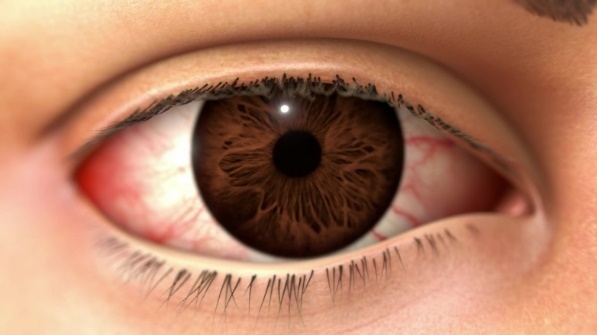
"Keratitis, a condition involving inflammation of the cornea, can be a complication for some patients with DED. Patients with severe keratitis from DED typically present with eye surface damage and inflammation that can sometimes feel like an irritating, itchy or burning sensation with possible blurry vision.
"Patients with severe inflammation of the cornea from DED are often managed with a number of different treatment options, which can be costly for a condition that requires ongoing management. A new subsidised treatment option will improve access for these patients," Prof. Watson said.
"Severe inflammation of the cornea from DED is complicated by a difficult cycle involving inflammation of the eye and damage to the eye surface. Treatment options targeting the immune system are therefore needed to manage these complications from DED, and break the cycle of inflammation," said Dr Margaret Lam, Optometrist, Head of Professional Services at George and Matilda Eyecare and Adjunct Senior Lecturer at the School of Optometry & Vision Science, UNSW.
"Artificial tears aim to provide symptom relief for DED, but don't address the underlying cause of severe corneal inflammation. In these cases, inflammation-reducing treatment options are required. Today's PBS listing of another treatment option for severe keratitis in adults with DED is therefore welcome news for ophthalmologists, optometrists and patients alike," Dr Lam said.
Executive Chair of The B Team Australasia, Lynette has spent the past 16 years living with severe corneal inflammation from DED. Soon after undergoing cataract surgery on both of her eyes in 2005, her eyes became extremely irritated, and she developed double vision. Little did she know at the time, her cataract surgery, coupled with a delayed diagnosis and her advancing age, would result in developing severe corneal inflammation from DED.
"I'm often unable to see things. For instance, I have to carry a magnifying glass when my eyes get blurry, or I develop double vision. Even at the supermarket, I have to carry a magnifying glass to read the food," said Lynette. "When people ask me to look at something for them at work, whether it is a document, or on-screen, depending on the health of my eyes at the time, I find it really difficult."
Lynette maintains it is important for Australian adults living with severe corneal inflammation from DED to have timely access to a range of treatment options. "Had my eye disease been detected earlier, it may not have had such an impact on my life," Lynette said.
Seqirus Head of Medical Affairs for the International Region, Dr Jonathan Anderson, Melbourne, said Seqirus is excited to be introducing the company's second PBS listed treatment option to the eye care market this year.
"Seqirus is committed to broadening access to eye care products to help address unmet clinical needs," said
Dr Anderson. "Today's PBS listing of Ikervis for severe keratitis in adults with DED will give Australians access to another treatment option that has long been available overseas."
About Ikervis
TGA approved in December 2020, Ikervis is a Schedule 4 (S4): Prescription Only Medicine. Ikervis is listed on the PBS from 1 October 2021 for severe keratitis in DED, for prescription by an optometrist or ophthalmologist and requires Authority Approval.
About Seqirus
Seqirus, a CSL company, is a leading provider of essential vaccines and pharmaceuticals. Having served Australia's healthcare needs for over a century, today we operate Australia's only local manufacturing facility for seasonal and pandemic influenza vaccines. Seqirus produces unique medicines in the National Interest, and also in licences a broad range of paediatric and adult vaccines and specialty pharmaceutical products. Visit www.seqirus.com.
Mel Kheradi, VIVA! Communications, m: 0421 551 257, e: melorin@vivacommunications.com.au
References:
1. Ikervis Approved Product Information.
2. The Pharmaceutical Benefits Scheme (PBS). Medicine Status - Ciclosporin. 2021 September 2021]; Available from: https://www.pbs.gov.au/medicinestatus/document/498.html.
3. Krolo, I., et al., Mask-Associated Dry Eye During COVID-19 Pandemic-How Face Masks Contribute to Dry Eye Disease Symptoms. Med Arch, 2021. 75(2): p. 144-148.
4. Barabino, S., A Narrative Review of Current Understanding and Classification of Dry Eye Disease with New Insights on the Impact of Dry Eye during the COVID-19 Pandemic. Ophthalmol Ther, 2021. 10(3): p. 495-507.
5. Amrane, M., et al., Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease - a randomised comparative study. J Fr Ophtalmol, 2014. 37(8): p. 589-98.
6. Leonardi, A., B. Flamion, and C. Baudouin, Keratitis in Dry Eye Disease and Topical Ciclosporin A. Ocular Immunology and Inflammation, 2017. 25(4): p. 577-586.
7. Baudouin, C., et al., A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol, 2017. 27(5): p. 520-530.
8. Stapleton, F., et al., TFOS DEWS II Epidemiology Report. The Ocular Surface, 2017. 15(3): p. 334-365.
Copyright 2021 ACN Newswire. All rights reserved. www.acnnewswire.com
source https://www.acnnewswire.com/press-release/english/69984/